3. Energetics of photoprocesses—electronically excited states A*
For practical reasons, it makes sense to consider light primarly as a form of energy. Processes involving light always include energy conversions in which light is involved as an energetic input (e.g. in solar cells and in photosynthesis) or output (e.g. in LEDs and in chemiluminescence) or as both (e.g. in fluorescence and phosphorescence). The “heart” of every photoprocess is the electronically excited state A* of a molecule or another atomic group:
"The 'photo' part of molecular photochemistry is a historical prefix and is now too restrictive. It is now clear that electronically excited states of molecules are the heart of all photoprocesses. The excited state is in fact an electronic isomer of the ground state."
- N. J. Turro, Modern Molecular Photochemistry, 1978
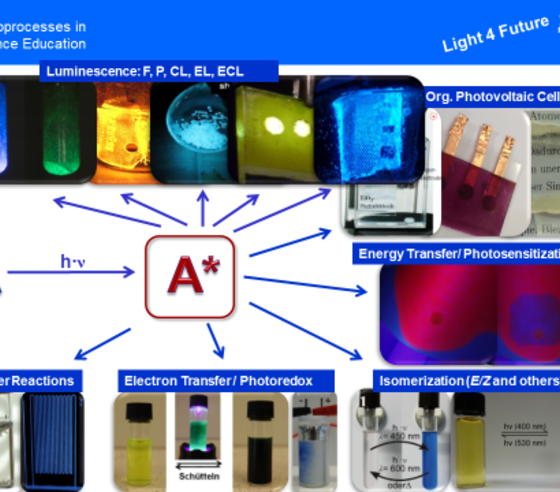
The excited state A* is usually generated by irradiating the ground state A with visible or UV light, but can also be generated by supplying a different form of energy, e.g. electrical energy. The deactivation of A*, i.e. its transition to the "old" ground state (ultimately no substance conversion) or a "new" one (formation of a new substance corresponding to a chemical reaction), can initiate a whole zoo of photoprocesses (see left picture).
These very different photoprocesses can be explored on the subpages experiments, videos, and models from this platform.
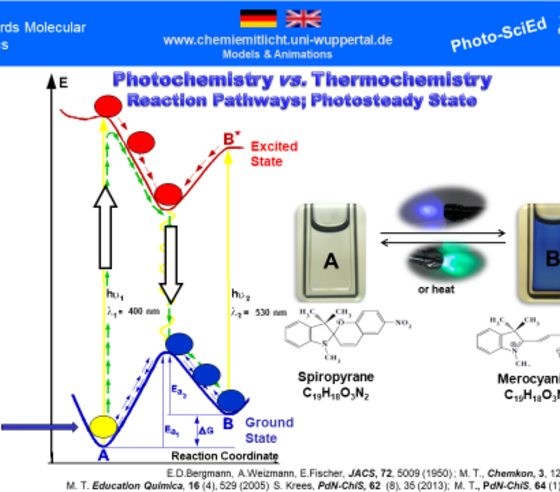
An important didactic challenge is to work out the fundamental difference between thermal and photochemical reactions. Experiments with the isomer pair spiropyrane/merocyanine offer excellent possibilities for demonstrating and investigating the phenomenon of photochromism in schools. Using the molecular switch spiropyrane/merocyanine the difference between thermal and photochemical reactions can be worked out on the phenomenological level. The observed reactions driven by irradiation and/or by heating can be explained using an energy diagram as shown above. The abscissa represents the “reaction coordinate” and the ordinate shows the energy content of the reacting system. molecule. Whereas the reacting system in a thermal reaction moves exclusively along the energy curve of the ground state, in a photochemical reaction it moves for part of the way along the curve of the excited state A*. Accordingly, the diagram above shows the reaction pathways of photochemical and thermal interconversions in the molecular switch spiropyran/merocyanine.
